Growth Trends in Toe Separator Products for Professional Footcare Suppliers
DONGGUAN, GUANGDONG, CHINA, November 24, 2025 /EINPresswire.com/ -- As awareness of personal wellness continues to rise worldwide, demand for foot health products has grown steadily, leading to notable expansion within several specialized segments of the broader footcare industry. Among these segments, the market for toe separators has shown particularly strong development. Dongguan Suscong Healthcare Co., Ltd., a manufacturer of toe separator products for footcare suppliers, established in 2011, is one of the companies contributing to this growth. Serving clients in more than 70 countries, Suscong has become a consistent supplier within the global footcare and orthopedic manufacturing network, focusing on toe separator products and other related solutions.
Global Footcare Market Outlook: Increasing Attention to Toe Alignment and Preventive Care
The footcare sector has undergone considerable change over the past decade, influenced by rising health consciousness, the expansion of recreational sports, and demographic shifts linked to aging populations. Today, the global market for footcare products is estimated at more than USD 6 billion. Its growth is supported by ongoing interest in preventive healthcare practices, comfort-enhancing products, and solutions designed for long-term musculoskeletal health.
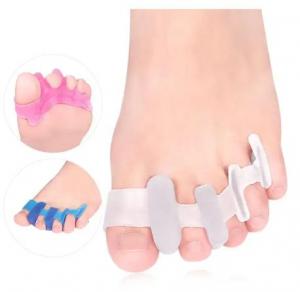
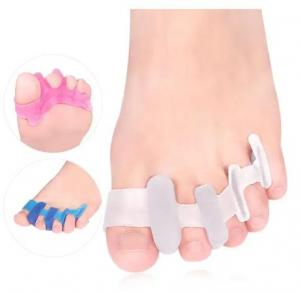
Within this landscape, toe separators—once regarded as a relatively narrow product category—have gained wider use in both consumer and clinical settings. Their applications now include support for toe alignment, reduction of discomfort, and assistance in managing conditions such as bunions, hammer toes, and plantar fasciitis. These items are made from materials such as medical-grade silicone, gel compounds, and soft TPE, aiming to provide users with cushioning, spacing, and ergonomic support. As a result, toe separators are now commonly found in orthopedic clinics, rehabilitation centers, beauty and nail-care facilities, and sports recovery programs.
Dongguan Suscong Healthcare Co., Ltd. has positioned itself as a manufacturer capable of supplying a broad portfolio of footcare items. Founded by General Manager Jeff Zhang, the company traces its origins to Zhang’s earlier involvement in handcrafted insole production through BDAC Company, established in Beijing in 2005. In 2011, he launched Suscong’s dedicated production facility in Dongguan City. The facility includes an R&D unit, multiple production lines, and a quality-control system designed to meet international sourcing requirements. Today, the company offers more than 500 product categories, ranging from orthopedic insoles and heel cushions to gel toe separators and arch-support solutions, supplying partners across Europe, North America, and Asia.
International Certifications and Compliance Standards
As global buyers place greater emphasis on verified product safety, ethical manufacturing, and environmental responsibility, Suscong Healthcare maintains a range of international certifications intended to demonstrate compliance with recognized industry standards.
Key certifications include:
SMETA Pillar 4: Addressing labor conditions, environmental management, and workplace safety.
WCA (Workplace Conditions Assessment): Evaluating working conditions and operational practices.
DUNS Registration: Providing global business identification and transparency.
Envisa Certification: Relating to environmental and manufacturing compliance.
CE Certification: Confirming conformity with European Union health and safety requirements.
GRS (Global Recycle Standard): Verifying the use of recycled or responsibly sourced materials.
FSC Certification: Ensuring responsible material sourcing in supply chains.
MHRA, KFDA, and SFDA Registrations: Meeting product compliance requirements in the UK, Korea, and China.
These certifications reflect the company’s adherence to internationally recognized manufacturing standards, aligning with regulations observed by many global healthcare retailers and distributors.
Participation in International Trade Exhibitions
To broaden its connections with global distributors and stay informed about new developments in materials and production technologies, Suscong Healthcare regularly participates in industry exhibitions and sourcing events. These platforms allow manufacturers to present new product lines, exchange technical knowledge, and engage with brands seeking OEM and ODM solutions.
Recent and upcoming exhibitions include:
Arab Health (Dubai): A major healthcare event featuring medical devices, rehabilitation supplies, and clinical innovations.
SOURCE FASHION (London): A venue that connects global buyers with manufacturing partners emphasizing traceability and responsible sourcing.
LIFESTYLE WEEK and GIFTEX TOKYO: Key trade fairs in Japan for lifestyle, wellness, and daily-use products.
TOKYO OEM/SOURCING EXPO: Focused on OEM and ODM collaboration opportunities.
INTEX & AFF International Textile Expo: Highlighting material technologies relevant to comfort products and footwear components.
Pakistan International Leather, Footwear, and Machinery Show (PMLS) and India International Leather Fair (IILF): Events focused on footwear materials, components, and supply-chain solutions.
Through these exhibitions, Suscong presents both its existing product lines and its developments in areas such as material optimization and customizable toe separator designs. These events also enable direct communication with distributors, supply-chain evaluators, and companies researching new footcare technologies.
Core Competencies and Operational Strengths
Suscong’s position within the global market is supported by several capabilities developed over years of manufacturing and product design:
1. Broad Product Range
The company provides more than 500 categories of footcare products, including toe separators, bunion-correction devices, heel cushions, arch supports, and sports-oriented insoles.
2. Research and Development
Its R&D unit focuses on ergonomic design, material performance, and adaptation to market trends. Product development often includes testing related to softness, elasticity, durability, and skin compatibility.
3. Quality Control Systems
The company employs a structured quality-management process aimed at maintaining consistency across production batches. Inspections typically include material testing, dimensional checks, and assessments of product durability.
4. Customization and OEM/ODM Services
Suscong offers customized specifications, including modifications to materials, hardness, dimensions, and packaging to accommodate brand-specific requirements.
5. Environmentally Considerate Manufacturing
The company incorporates recyclable materials and processes aligned with GRS and FSC standards, reflecting increasing market expectations for environmentally responsible production.
6. Global Supply-Chain Capability
With manufacturing partnerships and logistics channels across Asia and Europe, the company supports high-volume production and international shipping schedules.
Suscong’s products are used in professional contexts such as orthopedic clinics, rehabilitation centers, wellness facilities, beauty-care services, and sports recovery programs. Several distributors in Europe and Asia source private-label silicone and gel toe separators through the company for use in clinical recovery, daily comfort, and footwear-support applications. These collaborations underscore the company’s role as a supplier adaptable to diverse market needs.
Looking Ahead: Developments in Footcare Manufacturing
As the global demand for footcare solutions continues to expand, manufacturers are increasingly focused on material innovation, user comfort, and long-term product reliability. Dongguan Suscong Healthcare Co., Ltd. continues to invest in R&D, certification compliance, and process optimization to align with these industry directions. The company’s aim is to support footcare distributors, brands, and healthcare providers with products developed through consistent engineering, tested materials, and adherence to international standards.
Through participation in global exhibitions, cooperation with international partners, and ongoing improvements to manufacturing systems, Suscong continues to contribute to the evolving landscape of footcare products, particularly in the field of toe separators and related solutions.
For more information about its product range or cooperation inquiries, please visit the company’s official website:
https://www.suscong.com/
Global Footcare Market Outlook: Increasing Attention to Toe Alignment and Preventive Care
The footcare sector has undergone considerable change over the past decade, influenced by rising health consciousness, the expansion of recreational sports, and demographic shifts linked to aging populations. Today, the global market for footcare products is estimated at more than USD 6 billion. Its growth is supported by ongoing interest in preventive healthcare practices, comfort-enhancing products, and solutions designed for long-term musculoskeletal health.
Within this landscape, toe separators—once regarded as a relatively narrow product category—have gained wider use in both consumer and clinical settings. Their applications now include support for toe alignment, reduction of discomfort, and assistance in managing conditions such as bunions, hammer toes, and plantar fasciitis. These items are made from materials such as medical-grade silicone, gel compounds, and soft TPE, aiming to provide users with cushioning, spacing, and ergonomic support. As a result, toe separators are now commonly found in orthopedic clinics, rehabilitation centers, beauty and nail-care facilities, and sports recovery programs.
Dongguan Suscong Healthcare Co., Ltd. has positioned itself as a manufacturer capable of supplying a broad portfolio of footcare items. Founded by General Manager Jeff Zhang, the company traces its origins to Zhang’s earlier involvement in handcrafted insole production through BDAC Company, established in Beijing in 2005. In 2011, he launched Suscong’s dedicated production facility in Dongguan City. The facility includes an R&D unit, multiple production lines, and a quality-control system designed to meet international sourcing requirements. Today, the company offers more than 500 product categories, ranging from orthopedic insoles and heel cushions to gel toe separators and arch-support solutions, supplying partners across Europe, North America, and Asia.
International Certifications and Compliance Standards
As global buyers place greater emphasis on verified product safety, ethical manufacturing, and environmental responsibility, Suscong Healthcare maintains a range of international certifications intended to demonstrate compliance with recognized industry standards.
Key certifications include:
SMETA Pillar 4: Addressing labor conditions, environmental management, and workplace safety.
WCA (Workplace Conditions Assessment): Evaluating working conditions and operational practices.
DUNS Registration: Providing global business identification and transparency.
Envisa Certification: Relating to environmental and manufacturing compliance.
CE Certification: Confirming conformity with European Union health and safety requirements.
GRS (Global Recycle Standard): Verifying the use of recycled or responsibly sourced materials.
FSC Certification: Ensuring responsible material sourcing in supply chains.
MHRA, KFDA, and SFDA Registrations: Meeting product compliance requirements in the UK, Korea, and China.
These certifications reflect the company’s adherence to internationally recognized manufacturing standards, aligning with regulations observed by many global healthcare retailers and distributors.
Participation in International Trade Exhibitions
To broaden its connections with global distributors and stay informed about new developments in materials and production technologies, Suscong Healthcare regularly participates in industry exhibitions and sourcing events. These platforms allow manufacturers to present new product lines, exchange technical knowledge, and engage with brands seeking OEM and ODM solutions.
Recent and upcoming exhibitions include:
Arab Health (Dubai): A major healthcare event featuring medical devices, rehabilitation supplies, and clinical innovations.
SOURCE FASHION (London): A venue that connects global buyers with manufacturing partners emphasizing traceability and responsible sourcing.
LIFESTYLE WEEK and GIFTEX TOKYO: Key trade fairs in Japan for lifestyle, wellness, and daily-use products.
TOKYO OEM/SOURCING EXPO: Focused on OEM and ODM collaboration opportunities.
INTEX & AFF International Textile Expo: Highlighting material technologies relevant to comfort products and footwear components.
Pakistan International Leather, Footwear, and Machinery Show (PMLS) and India International Leather Fair (IILF): Events focused on footwear materials, components, and supply-chain solutions.
Through these exhibitions, Suscong presents both its existing product lines and its developments in areas such as material optimization and customizable toe separator designs. These events also enable direct communication with distributors, supply-chain evaluators, and companies researching new footcare technologies.
Core Competencies and Operational Strengths
Suscong’s position within the global market is supported by several capabilities developed over years of manufacturing and product design:
1. Broad Product Range
The company provides more than 500 categories of footcare products, including toe separators, bunion-correction devices, heel cushions, arch supports, and sports-oriented insoles.
2. Research and Development
Its R&D unit focuses on ergonomic design, material performance, and adaptation to market trends. Product development often includes testing related to softness, elasticity, durability, and skin compatibility.
3. Quality Control Systems
The company employs a structured quality-management process aimed at maintaining consistency across production batches. Inspections typically include material testing, dimensional checks, and assessments of product durability.
4. Customization and OEM/ODM Services
Suscong offers customized specifications, including modifications to materials, hardness, dimensions, and packaging to accommodate brand-specific requirements.
5. Environmentally Considerate Manufacturing
The company incorporates recyclable materials and processes aligned with GRS and FSC standards, reflecting increasing market expectations for environmentally responsible production.
6. Global Supply-Chain Capability
With manufacturing partnerships and logistics channels across Asia and Europe, the company supports high-volume production and international shipping schedules.
Suscong’s products are used in professional contexts such as orthopedic clinics, rehabilitation centers, wellness facilities, beauty-care services, and sports recovery programs. Several distributors in Europe and Asia source private-label silicone and gel toe separators through the company for use in clinical recovery, daily comfort, and footwear-support applications. These collaborations underscore the company’s role as a supplier adaptable to diverse market needs.
Looking Ahead: Developments in Footcare Manufacturing
As the global demand for footcare solutions continues to expand, manufacturers are increasingly focused on material innovation, user comfort, and long-term product reliability. Dongguan Suscong Healthcare Co., Ltd. continues to invest in R&D, certification compliance, and process optimization to align with these industry directions. The company’s aim is to support footcare distributors, brands, and healthcare providers with products developed through consistent engineering, tested materials, and adherence to international standards.
Through participation in global exhibitions, cooperation with international partners, and ongoing improvements to manufacturing systems, Suscong continues to contribute to the evolving landscape of footcare products, particularly in the field of toe separators and related solutions.
For more information about its product range or cooperation inquiries, please visit the company’s official website:
https://www.suscong.com/
Dongguan Suscong Healthcare Co., Ltd.
Dongguan Suscong Healthcare Co., Ltd.
+86 769 8707 9888
ssc@bdac.com
Visit us on social media:
Facebook
YouTube
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.